Abstract
Introduction
Patients with refractory or relapsed diffuse large B cell lymphoma (DLBCL) have very limited treatment options. Axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel are the Anti-CD-19 chimeric antigen receptor T-cell (CAR-T) therapy have been recently approved for refractory or relapsed DLBCL patients who have failed at least two lines of treatment. CAR-T therapy has significant adverse effects, most notably cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), limiting its use, particularly in the elderly population. In this meta-analysis, we analyzed the safety of CAR-T therapy in the elderly population compared to the younger patients with DLBCL.
Methods
Randomized controlled trials and observational studies in adult patients with DLBCL treated with anti-CD-19 CAR-T therapy were included in the meta-analysis. Case reports, case series, and review articles were excluded. We searched PubMed for studies published before 1st July 2021 using keywords "tisagenlecleucel," "axicabtagene ciloleucel," and "lisocabtagene maraleucel." The study selection process is shown in figure 1. Statistical analysis was performed with Comprehensive meta-analysis version 3. The fixed-effect model was used for pooled analysis. Heterogeneity across studies was analyzed using I2 statistics.
Results
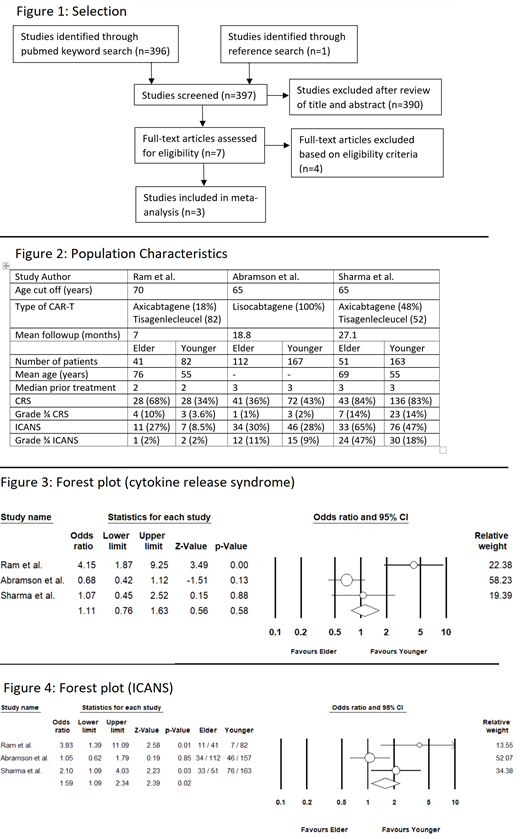
Three studies, comprising of 616 patients, were included in the analysis. Characteristics of the study population and the number of events in each study are shown in figure 2. Two studies used a cut-off of 65 years, while one study used a cut-off of 70 years for the older population. The mean age in the younger population was 55, while the mean age in the elderly population ranged from 69 to 76. Mean follow-up ranged widely in the three studies ranging from 7 months to 27.1 months.
Incidence of CRS ranged from 36% to 84% in the elderly population compared to 34% to 83% in younger patients. Grade 3 or 4 CRS ranged from 1 to 14% in the elder compared to 2 to 14% in younger patients. The pooled analysis shown in figure 3 revealed an odd ratio of 1.11 with a confidence interval ranging from 0.76 to 1.63 (p=0.58), implying there is no statistical difference between the two groups.
The incidence of ICANS ranged from 27% to 65% in elderly patients compared to 8.5% to 47% in younger patients. Grade 3 or 4 ICANS ranging from 2 to 47% in elderly patients compared to 2% to 18% in younger patients. The pooled analysis shown in figure 4 showed an odd ratio of 1.59 with a confidence interval ranging from 1.09 to 2.34 (p=0.02), suggesting the risk of ICANS is higher in older patients.
Conclusion
CRS and ICANS are the most common adverse effects limiting the use of CAR-T therapy in the elderly. Our analysis showed that age does not impact the risk of CRS in DLBCL patients treated with anti-CD19 CAR-T treatment. However, older patients are at a higher risk of ICANS as compared to younger, suggesting CAR-T therapy should be used with caution in patients older than 65 years of age, especially if they have baseline neurological impairment.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal